A Comparison of Glycaemic Control in Adults and Adolescents With Type 1 Diabetes When Using a Closed Loop Insulin Delivery System Overnight Versus Sensor-Augmented Pump With Low Glucose Suspend At Home: A Randomised Crossover Study (#159)
BACKGROUND: We compared glycaemic control with home use of night-time Android-based hybrid closed loop system (Android-HCLS) with sensor-augmented pump with low glucose suspend (SAP-LGS) in people with type 1 diabetes.
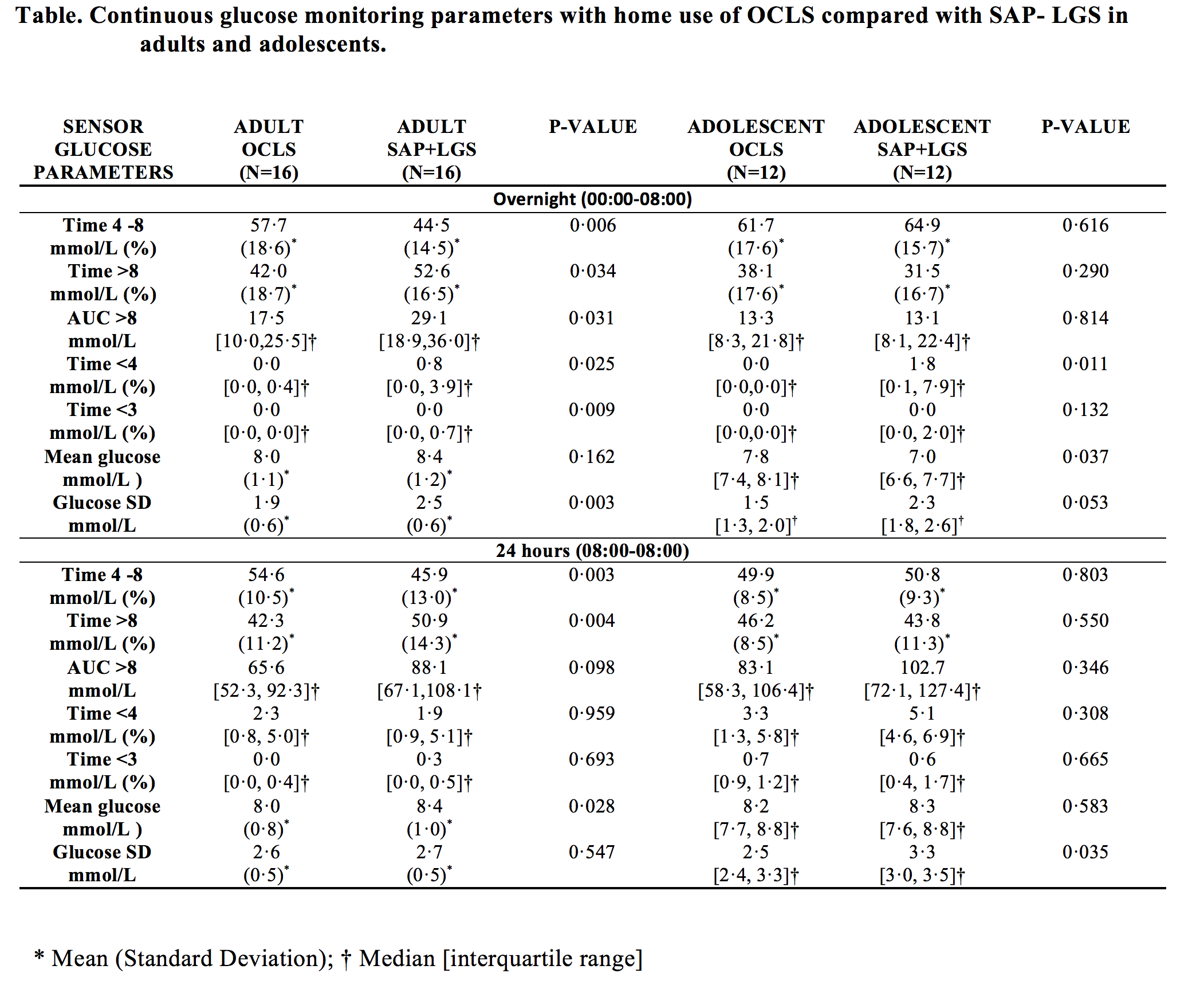
METHODS: An open-label, prospective, randomised, crossover study of 16 adults (mean [SD] age 42·1 [9·6] years) and 12 adolescents (15·2 [1·6] years) with type 1 diabetes was conducted. Randomisation was computer-generated, and allocation concealed until run-in completion. All participants completed four nights at home with Android-HCLS (proportional integral derivative with insulin feedback algorithm; Medtronic) and SAP-LGS. Primary outcome: percent continuous glucose monitoring (CGM) time (00:00-08:00hrs) within target range (4-8mmol/L). Secondary endpoints: percent CGM time above target (>8mmol/L); below target (<4mmol/L); glycaemic variability (SD) and symptomatic hypoglycaemia.
RESULTS: Using Android-HCLS, compared with SAP-LGS, adults had greater mean[SD] percent time within target range (57·7[18·6]% vs 44·5[14·5]%; p<0·006); less time above target (42·0[18·7]% vs 52·6[16·5]%; p=0·034); lower glycaemic variability (1·9[0·6] vs 2·6[0·6]; p=0·003) and less (median[IQR]) time below target (0·0[0·0, 0·4]% vs 0·80[0·0,3·9]%; p=0·025). In adolescents, time below target was lower with Android-HCLS vs SAP-LGS(0·0[0·0,0·0]% vs 1·8[0·1,7·9]%; p=0·011). Symptomatic hypoglycaemia was less (1 vs 10; p=0·007) in adolescents but not adults (5 vs 13; p=0·059).
CONCLUSIONS: Android-HCLS in both adults and adolescents reduced nocturnal hypoglycaemia and in adults improved overnight time in target range and treatment satisfaction compared with best contemporary treatment.